Embark on an enlightening journey into the fascinating realm of conservation of mass, a fundamental principle that governs the behavior of matter. This comprehensive guide, “Conservation of Mass Worksheet Answers,” unveils the intricacies of this concept, empowering you with a deeper understanding of the physical world.
Through a series of meticulously crafted explanations, real-life examples, and insightful discussions, this guide delves into the significance of conservation of mass in scientific disciplines. Discover how this principle shapes our understanding of chemical reactions, physical processes, and the very nature of matter itself.
1. Conservation of Mass Introduction

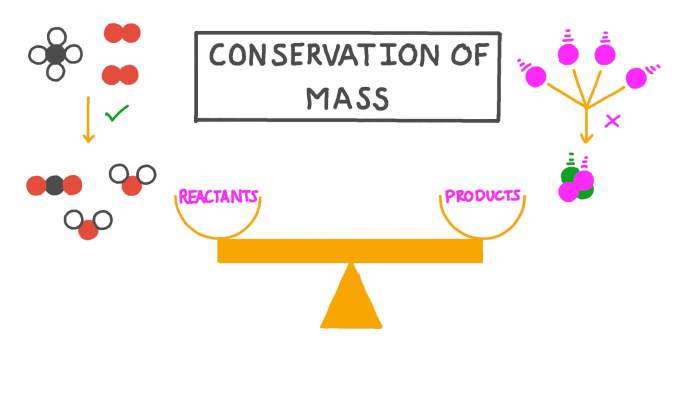
The conservation of mass is a fundamental law of nature that states that mass can neither be created nor destroyed in any chemical or physical change. In other words, the total mass of the reactants in a reaction will always be equal to the total mass of the products.
The conservation of mass is one of the most important laws in science, and it has been used to explain a wide variety of phenomena, from the behavior of gases to the formation of stars.
2. Conservation of Mass Calculations
Conservation of mass calculations are used to determine the mass of a product or reactant in a chemical reaction. To perform a conservation of mass calculation, you must know the masses of all the reactants and products in the reaction.
The following steps can be used to solve a conservation of mass problem:
- Write the balanced chemical equation for the reaction.
- Identify the reactants and products in the reaction.
- Determine the mass of each reactant and product.
- Use the conservation of mass law to calculate the mass of the unknown reactant or product.
It is important to note that conservation of mass calculations can only be performed for closed systems. A closed system is a system that does not exchange mass with its surroundings.
3. Conservation of Mass Applications: Conservation Of Mass Worksheet Answers
The conservation of mass has a wide range of applications in various fields, including chemistry, physics, and engineering.
- Chemistry:The conservation of mass is used to balance chemical equations and to calculate the mass of reactants and products in chemical reactions.
- Physics:The conservation of mass is used to explain the behavior of gases and to calculate the mass of objects in motion.
- Engineering:The conservation of mass is used to design and build machines and structures.
The conservation of mass is a fundamental law of nature that has a wide range of applications in science and engineering.
4. Conservation of Mass Experiments
There are a number of experiments that can be used to demonstrate the conservation of mass. One simple experiment is to weigh a piece of paper before and after it is burned.
Materials:
- Piece of paper
- Scale
Procedure:
- Weigh the piece of paper.
- Burn the piece of paper.
- Weigh the ashes.
Results:
The mass of the ashes will be less than the mass of the original piece of paper. This is because some of the mass of the paper was lost as gases during the burning process. However, the total mass of the paper and the ashes will be the same, which demonstrates the conservation of mass.
FAQs
What is the fundamental concept behind conservation of mass?
Conservation of mass states that the total mass of an isolated system remains constant, regardless of changes in state or composition.
How are conservation of mass calculations performed?
Conservation of mass calculations involve equating the initial mass of a system to the final mass, considering all inputs and outputs of matter.
What are common mistakes to avoid in conservation of mass calculations?
Common mistakes include neglecting to account for all substances involved, assuming complete reactions, and ignoring the role of energy in mass changes.