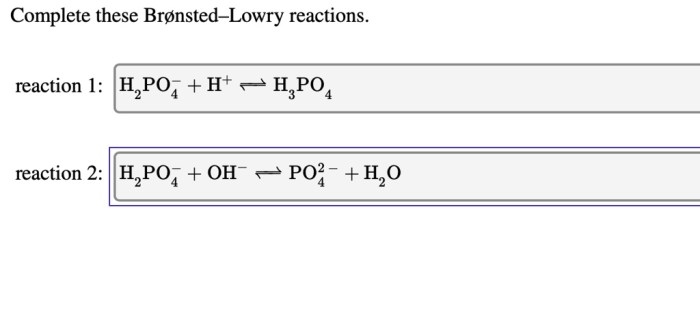
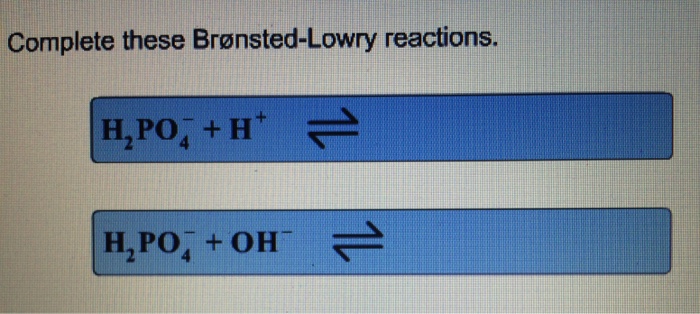
Complete these bronsted lowry reactions – Embarking on a journey to master Bronsted-Lowry reactions, we delve into the fundamental principles that govern acid-base interactions. These reactions, named after the renowned chemists Johannes Bronsted and Thomas Lowry, provide a comprehensive framework for understanding the behavior of acids and bases in various chemical systems.
Throughout this discourse, we will unravel the intricacies of Bronsted-Lowry acid-base reactions, exploring their mechanisms, applications, and significance in shaping the chemical landscape around us.
Bronsted-Lowry Acid-Base Reactions
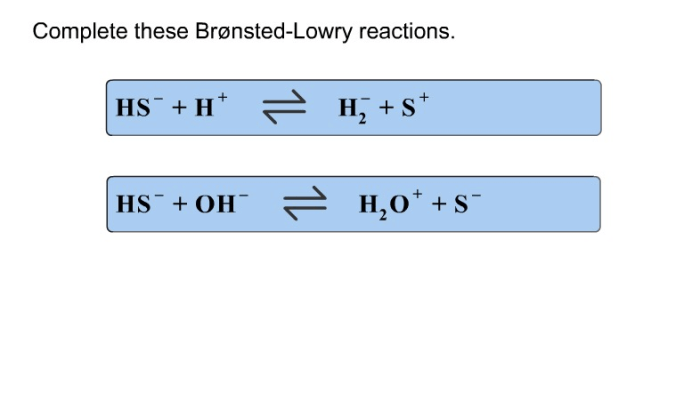
Bronsted-Lowry acid-base reactions are chemical reactions that involve the transfer of a proton (H +ion) from an acid to a base. In these reactions, an acid is a substance that can donate a proton, while a base is a substance that can accept a proton.
The role of protons in Bronsted-Lowry reactions is crucial as they are the species that are transferred between the acid and the base. When an acid donates a proton, it becomes its conjugate base, and when a base accepts a proton, it becomes its conjugate acid.
Conjugate Acid-Base Pairs, Complete these bronsted lowry reactions
In Bronsted-Lowry reactions, the acid and its conjugate base, as well as the base and its conjugate acid, form conjugate acid-base pairs. These pairs have the same number of atoms and electrons but differ in their protonation state.
Completing Bronsted-Lowry Reactions
To complete Bronsted-Lowry reactions, follow these steps:
- Identify the acid and the base in the reaction.
- Write the chemical equation for the reaction, showing the transfer of a proton from the acid to the base.
- Balance the chemical equation by adding coefficients to the reactants and products to ensure that the number of atoms of each element is the same on both sides of the equation.
Examples of Bronsted-Lowry Reactions
The following table provides examples of Bronsted-Lowry reactions:
| Reactants | Products | Conjugate Acid-Base Pairs |
|---|---|---|
| HCl + H2O | H3O+ + Cl– | HCl/Cl–, H2O/H3O+ |
| NH3 + H2O | NH4+ + OH– | NH3/NH4+, H2O/OH– |
| CH3COOH + H2O | CH3COO– + H3O+ | CH3COOH/CH3COO–, H2O/H3O+ |
These reactions play a significant role in various real-life applications, such as acid-base titrations, buffer solutions, and biological processes.
Applications of Bronsted-Lowry Reactions
Bronsted-Lowry reactions have numerous applications, including:
- Acid-Base Titrations:These reactions are used to determine the concentration of an unknown acid or base by reacting it with a known concentration of a base or acid, respectively.
- Buffer Solutions:Bronsted-Lowry reactions are used to create buffer solutions, which resist changes in pH when small amounts of acid or base are added.
- Biological Processes:Bronsted-Lowry reactions play a crucial role in biological processes, such as enzyme catalysis, where the transfer of protons facilitates the chemical reactions necessary for life.
Common Queries: Complete These Bronsted Lowry Reactions
What is the key concept behind Bronsted-Lowry acid-base reactions?
Bronsted-Lowry acid-base reactions focus on the transfer of protons between species, where an acid donates a proton to a base, resulting in the formation of conjugate acid-base pairs.
How can I identify the acid and base in a Bronsted-Lowry reaction?
The acid is the species that donates a proton, while the base is the species that accepts a proton. Typically, acids have a tendency to lose protons, while bases have an affinity for gaining protons.
What are some real-life applications of Bronsted-Lowry reactions?
Bronsted-Lowry reactions play a crucial role in acid-base titrations, buffer solutions, and various biological processes, including enzyme catalysis and maintaining the pH balance in living organisms.